Introduction
Alkalinity is the water’s capacity to resist changes in pH that would make the water more acidic. This capacity is commonly known as “buffering capacity.” For example, if you add the same weak acid solution to two vials of water – both with a pH of 7, but one with no buffering power (e.g. zero alkalinity) and the other with buffering power (e.g. an alkalinity of 50 mg/l), – the pH of the zero alkalinity water will immediately drop while the pH of the buffered water will change very little or not at all. The pH of the buffered solution would change when the buffering capacity of the solution is overloaded.
Alkalinity refers to the capability of water to neutralize acid. This is really an expression of buffering capacity. A buffer is a solution to which an acid can be added without changing the concentration of available H+ ions (without changing the pH) appreciably. It essentially absorbs the excess H+ ions and protects the water body from fluctuations in pH.
The alkalinity of natural water is determined by the soil and bedrock through which it passes. The main sources for natural alkalinity are rocks which contain carbonate, bicarbonate, and hydroxide compounds. Borates, silicates, and phosphates also may contribute to alkalinity. Limestone is rich in carbonates, so waters flowing through limestone regions or bedrock containing carbonates generally have high alkalinity – hence good buffering capacity. So water that comes in contact with limestone will contain high levels of both Ca++ and CO32- ions and have elevated hardness and alkalinity. Conversely, areas rich in granites and some conglomerates and sandstones may have low alkalinity and, therefore, poor buffering capacity.
Environmental Significance
- Alkalinity is important for fish and aquatic life because it protects or buffers against rapid pH changes. Living organisms, especially aquatic life, function best in a pH range of 6.0 to 9.0. Alkalinity is a measure of how much acid can be added to a liquid without causing a large change in pH. Higher alkalinity levels in surface waters will buffer acid rain and other acid wastes and prevent pH changes that are harmful to aquatic life.
- Large amount of alkalinity imparts bitter taste in water
- The principal objection of alkaline water is the reactions that occur between alkalinity and certain cations in waters. The resultant precipitate can corrode pipes and other accessories of water distribution systems
- Wastewaters containing excess caustic (hydroxide) alkalinity are not to be discharged into natural water bodies or sewers
- Alkalinity as carbonate and bicarbonate of saline water is very important in tertiary recovery process for recovering petroleum. Alkaline water offers better wetting to the formation rock and improve oil release. As an additional benefit, ions that provide alkalinity absorb on rock surfaces occupying adsorption sites and decrease the loss of recovery chemical by adsorption.
- The alkalinity value is necessary in the calculation of carbonate scaling tendencies of saline waters.
- The alkalinity acts as a pH buffer in coagulation and lime-soda softening of waters.
- In wastewater treatment, alkalinity is an important parameter in determining the amenability of wastes to the treatment process and control of process such as anaerobic digestion, where bicarbonate alkalinity, total alkalinity, and any fraction contributed by volatile acid salts become considerations.
Objective(s) of the Experiment
The test is carried out in order to determine the type and amount of alkalinity present in water
Apparatus and Reagents
Apparatus
- Burette (on the burette stand)
- Conical Flask
- Measuring Cylinder
- Funnel
- Beakers
Reagents
- Phenolphthalein Indicator
- Methyl Orange Indicator
- H2SO4 of strength N/50
Procedures
- First fill the burette with H2SO4 and let some drops out to remove the bubbles
- Using the measuring cylinder, measure 100ml of the sample and pour it inside the conical flask.
- Add 3-4 drops of phenolphthalein to the sample in the flask and shake the content to mix. If the colour changes to pink, you can proceed to item 4. But if not, proceed to item (a).
- Add 1 drop of methyl orange indicator and shake the content. If there is a light orange colour, this implies that there is no alkalinity present in the sample.
- If the colour changes to pink, you can then titrate the sample against the H2SO4 solution in the burette.
- Note down the initial reading of the burette, and place the conical flask under it.
- Open the cork of the burette and let the solution drop slowly and continue shaking until the pink colour disappears. Note down the reading of the burette at which this happen; and calculate the volume of H2SO4 used. That is final reading – initial reading. (This is recorded under phenolphthalein indicator. Check Table 1 below).
- Now, add 1 drop of methyl orange to the same sample and continue titration until appearance of light orange colour. (This is recorded under methyl orange indicator. Check Table 2 below).
- Note down the final reading at which this happens and calculate the volume of H2SO4 used.
- Repeat these procedures using the same volume of sample 3 to 4 times until a concordant value of volume used is gotten.
Results and Calculations
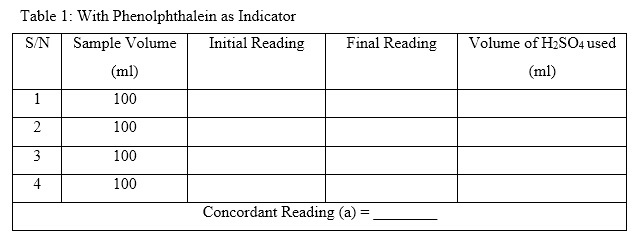
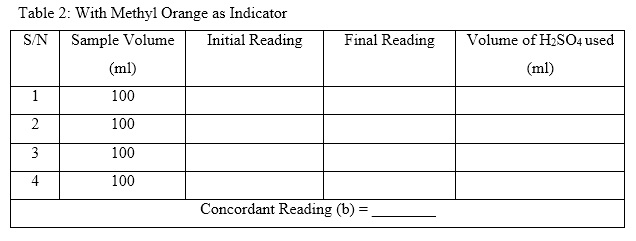
- Total volume of standard H2SO4 used for titration (c) = (a) + (b)
- Phenolphthalein Alkalinity,
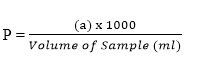
- Total Alkalinity,
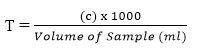
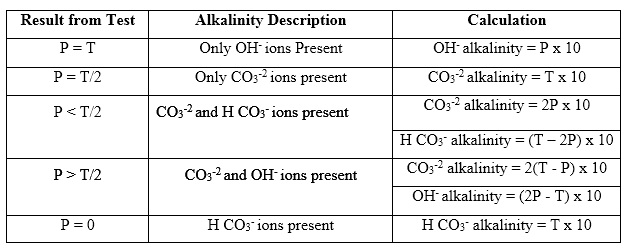
There are five combinations for P and T as shown in the table below: