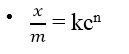Introduction
Adsorption is the process of transferring material from a fluid phase to a solid phase. It is a separation process in which some materials; (adsorbate) is concentrated from a bulk vapor or liquid phase on to the surface of a porous solid (adsorbent).The adsorption of acetic acid on charcoal is studied using both the Freundlich isotherm and the Langmuir isotherm. The amount of acetic acid (adsorbate) adsorbed per gram of charcoal (adsorbent) will depend on the surface area of the charcoal, the temperature of the solution, and the adsorbate concentration in solution. The adsorption will be followed by titrating the acetic acid not adsorbed by the charcoal, then determining the amount adsorbed by difference.
Isotherms (plots of moles of adsorbate adsorbed per gram of adsorbent versus solution concentration) will be constructed, and then compared with three models: Freundlich, Langmuir, and Amire. In this article, in order to develop understanding of adsorption acetic acid on charcoal and how the amount of acetic acid adsorbed varies with its concentration, this study is carried out using two different adsorption isotherms which are Freundlich and Langmuir isotherms.
Adsorption as a phenomenon is useful in industries for many practical applications, some of which are use of charcoal for decolourisation sugar solutions, removal of gas odours ( i.e. as gas mask), recovery of solvent vapours. Purifications of water and wastewater and also for various uses in chromatography.
Theory
Physisorption or physical adsorption is a type of adsorption in which the adsorbate adheres to the surface only through Van-der-Waals (weak intermolecular) interactions, which are also responsible for the non-ideal behaviour of real gases.
Chemisorption is a type of adsorption whereby a molecule adheres to a surface through the formation of a chemical bond, as opposed to the Van der Waals forces which cause physisorption. Adsorption is usually described through isotherms, that is, functions which connect the amount of adsorbate on the adsorbent, with its pressure (if gas) or concentration (if liquid).One can find in literature several models describing process of adsorption, namely Freundlich isotherm, Langmuir isotherm, etc.
Reagents and Apparatus
1. Charcoal
2. 0.15 M Acetic Acid
3. 0.03M Acetic Acid
4. Distilled Water
5. 0.1 M NaOH
6. Phenolphthalein Indicator
7. Refrigerator
8. Sets of Erlenmeyer Flasks
Procedures
1. Place approximately 1 g (weighed accurately to the nearest 0.001 g) of charcoal into each of seven dry Erlenmeyer flasks.
2. Prepare solutions of 0.15, 0.12, 0.09, 0.06, 0.03and 0.015 M acetic acid. These solutions can be made by diluting a stock solution of 0.15 M acetic acid.
3. Add 100 mL of a different acetic acid solution to each flask, with100 mL of distilled water to the seventh. An eighth flask should contain 0.03M Acetic acid, but no charcoal.
4. The flasks should be tightly stoppered, then shaken periodically for thirty minutes, and allowed to equilibrate for several hours (preferably overnight) in a water bath maintained at a constant temperature.
5. Repeat the procedure above on a second set of Erlenmeyer flasks but place them in the refrigerator to equilibrate at a lower temperature. Make sure to measure both equilibration temperatures.
6. After equilibration, the samples are filtered (discard the first 10 mL of filtrate). Titrate two 25 mL aliquots of each sample with a standardized 0.1 M NaOH solution using phenolphthalein as an indicator.
Results and Calculations
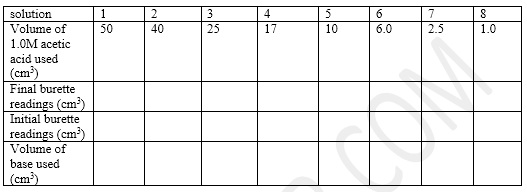
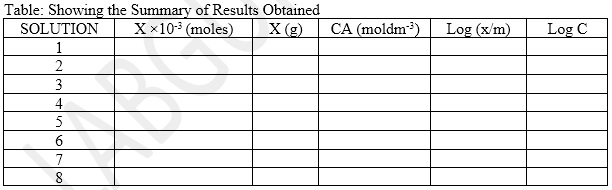
- The result is tabulated as in the table below:
- To calculate the concentration of the diluted solutions;
- Using the dilution formula,
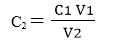
- Where:
C1 is concentration of stock solution = 1.0M
V1 is volume of acid used =
C2 is concentration of the diluted solution = ?
V2 is volume of volumetric flask used = 100ml - Calculate the concentration C2 for V1 = 50mL, 40 mL, 25 mL, 17 mL, 10 mL, 6 mL, 2.5 mL, and 1.0 mL
- Calculate the Number of Moles of Acetic Acid Adsorbed (H) for Each of the Solutions
Where
Equation for the reaction: CH3COOH + NaOH → CH3COONa + H2O
Molar mass of CH3COOH = (12×1) + (1×3) + 12 + (16×2) + 1 = 60g/mol - Using the relation
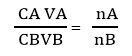
- Where:
CB = Concentration of titrant in mol/dm3 = 0.1mol/dm3
VB = the volume of base used in dm3
CA = Concentration of acetic acid after adsorption
VA = Volume of Acetic acid in dm3 nA = nB = 1 - Thus, moles of acetic acid absorbed, X for all eight solutions will be calculated as follows:
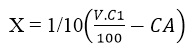
- Where
V = Volume of 1.0M acetic acid used (cm3) = 50mL, 40 mL, 25 mL, 17 mL, 10 mL, 6mL, 2.5 mL, and 1.0 mL respectively.
C1 = 1.0M.
CA is as calculated from above
FREUNDLICH’S ADSORPTION ISOTHERM
Where
X is as calculated above (in moles)
X (in grams) = (60g/mol) x (Xmoles)
CA is as calculated above
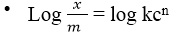
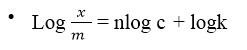
Plot a graph of log x/m against log C. Comparing the above equation with the equation of a straight line i.e. y = mx + c, the plot of logx/m against log C should give a straight line with:
- Slope = n
- Intercept = logk
- Slope = y1 – y2 / x1 – x2
LANGMUIR’S ADSORPTION ISOTHERM
Where m = 1 so it is mc/x = c/x directly.
• x/m = ac/1+bc
Rearranging the equation above, we have
• x(1+bc) = ac . m
• 1 + bc/ a = mc/a
• mc/x = 1/a + (b/a)c
Plot a graph of mc/x against c. When compared with the equation of a straight line, y = mx + c implies that the plot of mc/x against c should give a straight line graph with slope = b/a and intercept 1/a
Discussion and Conclusion
From the experimental result, you should be able to determine if the theory of adsorption of acetic acid is indeed dependent on concentration; and also, determine which adsorption isotherm is the best fit for the experimental data.
References
- Adamson, A. W. (1990). ‘Physical Chemistry of Surfaces, 5th Ed., John Wiley & Sons, New York.
- Shoemaker et al (1981). ‘Experiments in Physical Chemistry 4th Edition’, page 332‐336, McGraw‐Hill.
- www.fpharm.uniba.sk. ‘5-Determination of Adsorption Isotherm of Acetic Acid on Charcoal’
Report - Adsorption of Acetic Acid on Charcoal (0 downloads )
Procedure - Adsorption of Acetic Acid on Charcoal (0 downloads )