Introduction
The main cause of water hardness is dissolved salts of calcium and magnesium. Additionally, the other ions like Strontium, Iron, Barium and Manganese also contribute to water hardness. Traditionally, total hardness is measured by the amount of soap that is required to produce leather. Hardness affects the amount of soap that is needed to produce foam or lather. Hard water requires more soap, because the calcium and magnesium ions form complexes with soap, preventing the soap from foaming. Hard water can also leave a film on hair, fabrics, and glassware. Hardness of the water is very important in industrial uses, because it forms scale in heat exchange equipment, boilers, and pipes. Some hardness is needed in plumbing systems to prevent corrosion of pipes.
To determine the Total Hardness of Water, EDTA (Ethylenediamine tetra acetic acid) is the easiest method because EDTA reacts with all metal without alkali metal and the proportion is 1:1. EDTA forms colorless stable complexes with Ca2+ and Mg2+ ions present in water at pH = 9 – 10. Erichrome black – T dye (E.B.T) is used for this test.
At pH 10, EDTA forms colorless, water soluble stable complexes with calcium and magnesium ions. When the indicator Erichrome black T dye is added into the hard water, then the indicator forms unstable complex with calcium and magnesium ions and the solution turn into wine red. If there is no hardness the color becomes blue which is original color of indicator. Now, when this solution is titrated against EDTA, then the calcium and magnesium ions started to form a stable metal-EDTA complex. After all the free calcium and magnesium ions are consumed, the EBT is replaced by EDTA from the unstable complex and liberates the free Erichrome Black-T. Then the water color change from wine red to blue that indicates the end point.
Reactions
The reactions that take place during the titration are:
- Ca2+ + EDTA4- → CaEDTA2-
- Mg2+ + EDTA4- → MgEDTA2-
Types of Hardness
There are two types of hardness:
- The temporary hardness: This is due to the presence of Ca(HCO3) or Mg(HCO3) or both in the water.
- Permanent hardness: This is due to the presence of sulphates, chlorides and nitrates of calcium and magnesium (as like CaCl2, CaSO4, MgCl2 and MgSO4) in water.
To determine the Total Hardness of Water with EDTA method, an inorganic acid is initially added to convert temporary hardness into permanent hardness. Ca(HCO3)2 + 2HCl → CaCl2 + H2O + 2CO2
Objective(s) of the Experiment
This test is carried out in order to estimate the amount of total hardness present in the given sample of water by EDTA titration method.
Equipments and Materials Needed
Apparatus:
- Conical Flask
- Funnel
- Burette
- Sand
- Beaker
- Pipette
- Graduated cylinder
- Hot plate stirrer
- Wash bottle
- Spatula
Chemicals:
- Buffer solution
- Inhibitor
- Eriochrome black T indicator (blue color)
- NaOH
- Standard EDTA Solution 0.01M
- Magnesium sulphate
Safety Precaution
Avoid skin contact with chemicals. Clothing contaminated with NaOH solution should be carefully removed. Spillage adhering to skin should be immediately washed with plenty of water.
Procedures
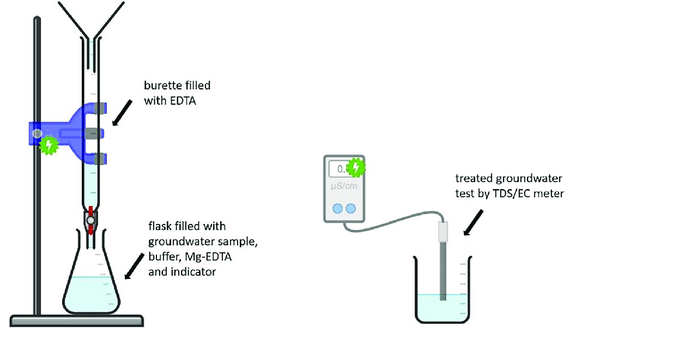
- Pipette 50ml of water sample into a conical flask.
- Add 2-3ml 1:1 HCl and boil for 2 min.
- Cool the solution and neutralized with the dilute NaOH.
- Add 1-5ml NH4OH/NH4Cl buffer solution. The pH should be 10. Check the pH with standardize pH meter. (you can also add an Inhibitor, after the buffer solution)
- Add 2-3 drops of 0.1M Mg-EDTA solution and 3-4 drops Erichrome Black T indicator. Then, shake well so that the color becomes wine red.
- Rinse a clean, dry burette with a small amount of EDTA solution first, then fill the burette with standardized 0.01M EDTA solution. Record the initial burette reading and titrate the water sample with this standard solution.
- At the end point the color of the solution turns into blue from wine red. Titrate carefully near the endpoint.
- Take the final burette reading, record as V1 mL.
- For more accurate result, you can run a blank titration. Record as V2 mL.
- Repeat the titration to obtain two or three concordant results.
Results and Calculations
Tabulate the readings as shown below:
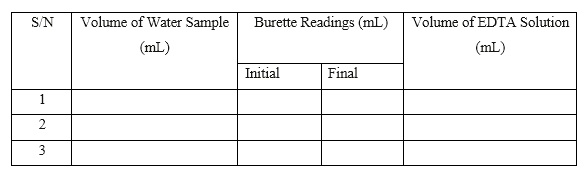
- Average Titre V = (V1 + V2 + V3)/3 = _________
- For blank titration, the volume of EDTA required by sample water, V = (V1-V2)ml
- The total hardness (temporary + permanent) can be calculated by using the following formula:
- Total Hardness = Calcium hardness + Magnesium hardness
- 1mL 0.01M EDTA ≡ 0.001001g CaCO3
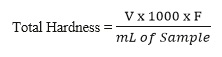
- Where V = Volume of EDTA used (ml)

References
- Student Handout. ‘Hardness of Water by EDTA Titration’ Other Learning Activity (6).
- VITS Engineering Chemistry Lab Manual. ‘Estimation of Hardness of Water by EDTA Method’.
- Hard and Soft Water. ‘Determination of Total Hardness of Water’ http://hardsoftwater.com/determination-of-total-hardness-of-water/ Assessed on March 10, 2018.
- Titrations.info. ‘Complexometric titration : EDTA – determination of water hardness’ www.titrations.info/EDTA-titration-water-hardness . Assessed on March 13, 2018.
- CVE 495 ”Environmental Engineering Lab Notes”.