Introduction
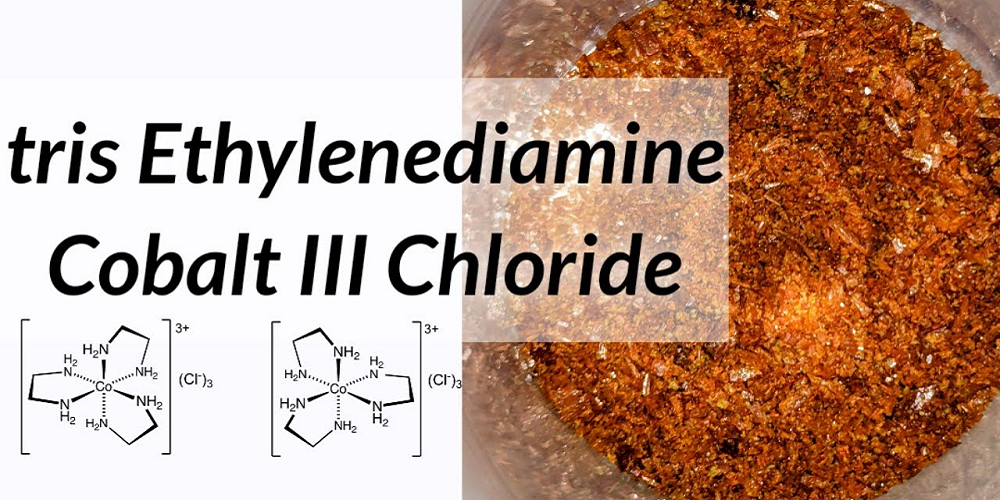
Tris(Ethylenediamine)cobalt(III) chloride is coordination compound with the formula [Co(en)3]Cl3. Ethylenediamine coordinates with the cobalt ion through both nitrogen atoms, the five membered chelate rings that are thus formed are very stable.
Aim of the Experiment
To synthesize tris(Ethylenediamine)cobalt(III) chloride and calculate the percent yield.
Apparatus and Reagents Needed
- CoCl2.6H2O
- Ethylenediamine dihydrochloride
- Conc. HCl
- Water aspirator
- Weighing balance
- Hot plate
- Ice bath
- Beaker
- Water aspirator
- Vacuum filtration setup
- Sample bottle
- Stirring rod
Procedures
- Weigh 6 g of CoCl2.6H2O to a beaker and add 25 mL of water and stir till fully dissolved, add 13 g of ethylenediamine dihydrochloride (H2NCH2CH2NH2.2HCl) to the solution then bubble in air into the beaker for 3-4 hours.
- Heat the solution until a crust begins to form over the surface, add 5 mL of concentrated HCl and 10 mL of ethanol and cool the solution in an ice bath.
- After cooling, filter out the crystals and wash with ethanol and dry.
Results and Calculations
- Calculate the relative formula masses and number of moles of CoCl2.6H2O, H2NCH2CH2NH2.2HCl used in the experiment.
- Calculate the theoretical yield in grams of [Co(en)3]Cl3.
- Use the recorded masses to derive the actual yield of the product.
- Use the theoretical yield and actual yield to calculate the percent yield.
cobalt(iii)chloride-pic1.jpg)
References
- Wikipedia (2018), “Tris(ethylenediamine)cobalt(III) chloride”. Retrieved from http://en.m.wikipedia.org/wiki/Tris(ethylenediamine)cobalt(III)_chloride (accessed 9 February 2018).
- “Synthesis, Optical Resolution and Derivatization of Co(en)33+”, retrieved from http://chemistry.bd.psu.edu/jircitano/Optical3.pdf (accessed 9 February 2018).