Effect of Heat Treatment on the Microstructure of Low Carbon Steel
Introduction
Steel
A Steel is a hard, tough metal composed of iron, alloyed with various small percentage of carbon and often variously with other metals such as nickel, chromium, manganese, etc. But to adequately defining a steel: the principal portion of the definition for steel is “an iron base alloy, malleable in some temperature range as initially cast, containing manganese, usually carbon, and often other alloying elements. In carbon steel and low-alloy steel, the maximum carbon is about 2.0%; in high-alloy steel, about 2.5%. The dividing line between low-alloy and high-alloy steels is generally regarded as being at about 5% metallic alloying elements
Fundamentally, all steels are mixtures, or more properly, alloys of iron and carbon. However, even the so-called plain-carbon steels have small, but specified, amounts of manganese and silicon plus small and generally unavoidable amounts of phosphorus and sulfur. The carbon content of plain-carbon steels may be as high as 2.0%, but such an alloy is rarely found. Carbon content of commercial steels usually ranges from 0.05 to about 1.0%.
Heat Treatment
Engineering properties are modified by heat treatment processes so that structural components are able withstand specified operating conditions and have desired useful life.
Heat treatment is the heating and cooling of metals to change their physical and mechanical properties, without letting it change its shape. Heat treatment could be said to be a method for strengthening materials but could also be used to alter some mechanical properties such as improving formability, machining, etc. The most common application is metallurgical but heat treatment can also be used in manufacture of glass, aluminum, steel and many more materials. The process of heat treatment involves the use of heating or cooling, usually to extreme temperatures to achieve the wanted result. It is very important manufacturing processes that can not only help manufacturing process but can also improve product, its performance, and its characteristics in many ways.
The manipulation of heat treatment response is a prime reason for adding alloying elements to steels. An appreciation of the thermal behavior, with the accompanying microstructural changes, is fundamental to the understanding of heat treatment and the mechanical properties so generated.
Why Heat Treat Metals
We heat treat metals in an attempt to optimize the mechanical and physical properties for a given application. As many heat treatments are applied to soften metal in order to allow metal working operations such as deep drawing, cold forging and machining.
Where increased strength and wear resistance is required, hardening and tempering treatments are given. Extremely hard steels find applications in cutting tools where highly defined edges must be maintained and heat treatment of these steels is a critical operation. Hard surfaces with ductile base material may be developed by heat treatment.
There are also the solution heat treatments and ageing processes designed to increase the strength of some non-ferrous metals and precipitation hardening steels. Heat treatment is a significant industry and forms a basic part of the industrial infrastructure of countries.
Terms Used in Heat Treatment
- Hardening:is a process of increasing the metal hardness, strength, toughness, fatigue resistance. It involves heating of steel, keeping it at an appropriate temperature until all pearlite is transformed into austenite, and then quenching it rapidly in water or oil.
- Strain hardening (work hardening) – strengthening by cold-work (cold plastic deformation).
- Grain size strengthening (hardening) – strengthening by grain refining.
- Solid solution hardening – strengthening by dissolving an alloying element.
- Dispersion strengthening – strengthening by addition of second phase into metal matrix.
- Annealing is a heat treatment procedure involving heating the alloy and holding it at a certain temperature (annealing temperature), followed by controlled cooling.
- Quenching involves heating the metal to high temperatures, and then cooling in water or oil or by undergoing a forced-air treatment.
- Tempering involves heating steel that has been quenched and hardened for an adequate period of time so that the metal can be equilibrated.
- Normalizing involves heating steel, and then keeping it at that temperature for a period of time, and then cooling it in air. The resulting microstructure is a mixture of ferrite and cementite which has a higher strength and hardness, but lower ductility.
Benefits of Steel Heat Treatment
- It aids in the manufacturing process
- The right heat treatment will alter both physical and mechanical properties of your material
- It is used to relieve stresses, making the steel easier to machine or weld
- It is used in processes such as hot forming or after welding, where stresses may have been built up over time.
- Increase in overall lifetime of the part
- Heat treatments are applied to soften metal in order to allow metal working operations such as deep drawing, cold forging and machining.
- Where increased strength and wear resistance is required, hardening and tempering treatments are given
- To relieve internal stresses, which are set up in the metal due to cold or hot working.
- To soften the metal.
- To improve hardness of the metal surface.
- To improve machinability.
- To refine grain structure
- To improve mechanical properties like tensile strength, ductility and shock resistance, etc.
- To improve electrical and magnetic properties.
- To increase the resistance to wear, tear, heat and corrosion
Detailed Note on Quenching
Quenching is the rapid cooling of a work piece in water, oil or air to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as phase transformations, from occurring. It does this by reducing the window of time during which these undesired reactions are both thermodynamically favorable, and kinetically accessible; for instance, quenching can reduce the crystal grain size of both metallic and plastic materials, increasing their hardness.
In metallurgy, quenching is most commonly used to harden steel by introducing martensite, in which case the steel must be rapidly cooled through its eutectoid point, the temperature at which austenite becomes unstable. In steel alloyed with metals such as nickel and manganese, the eutectoid temperature becomes much lower, but the kinetic barriers to phase transformation remain the same. This allows quenching to start at a lower temperature, making the process much easier. High speed steel also has added tungsten, which serves to raise kinetic barriers and give the illusion that the material has been cooled more rapidly than it really has. Even cooling such alloys slowly in air has most of the desired effects of quenching.
Extremely rapid cooling can prevent the formation of all crystal structure, resulting in amorphous metal or "metallic glass"
Process of Quenching
The process of quenching is a progression, beginning with heating the sample. Most materials are heated to between 815 and 900 °C (1,500 to 1,650 °F), with careful attention paid to keeping temperatures throughout the work piece uniform. Minimizing uneven heating and overheating is key to imparting desired material properties.
The second step in the quenching process is soaking. Work pieces can be soaked in air (air furnace), a liquid bath, or a vacuum. The recommended time allocation in salt or lead baths is up to 6 minutes. Soaking times can range a little higher within a vacuum. As in the heating step, it is important that the temperature throughout the sample remains as uniform as possible during soaking.
Once the work piece has finished soaking, it moves on to the cooling step. During this step, the part is submerged into some kind of quenching fluid; different quenching fluids can have a significant effect on the final characteristics of a quenched part. Water is one of the most efficient quenching media where maximum hardness is desired, but there is a small chance that it may cause distortion and tiny cracking. When hardness can be sacrificed, mineral oils are often used. These oil based fluids often oxidize and form a sludge during quenching, which consequently lowers the efficiency of the process. The quenching velocity (cooling rate) of oil is much less than water. Intermediate rates between water and oil can be obtained with a purpose formulated quenchant, a substance with an inverse solubility which therefore deposits on the object to slow the rate of cooling.
Oil Quenching
Heat treated metal parts perform better. They resist wear and maintain their form while under pressure. An oven heats metal components to extreme temperatures and then submerged in a liquid bath for rapid cooling. Water was the traditional quenching medium. However, oil quenching produces superior quality.
The Advantages of Oil Quenching
Oil does not boil as quickly as water. This allows the parts to absorb more initial heat and cool for longer periods of time. It is a superior quenching heat treatment, and including certain additives in the oils customize results.
The process begins with heat. Metal parts are placed in an oven. Temperatures can reach as high as 2000 degrees. Once the desired heat has been achieved, the parts are transferred to a submersion tank. The metal is coated in the oils and undergoes three phases of cooling:
- Vapor Blanket Phase – High heat vaporizes oil forming an insulating cloud around the metal.
- Nucleate Boiling – Vapors dissipate, and heat energy transfers to the oil from the metal.
- Convection – The oil is heated to its boiling point and heat energy quickly dissipates.
Ideally, the vapor phase will be as short as possible. The vapors created by the searing hot metals act as an insulator that resists cooling. The prolonged period of nucleate boiling is the key advantage of oil quenching services over traditional hardening methods. The longer the parts remain in the second phase of the cooling process, the better the result.
Oil has a much higher boiling point than water. The metals do not reach the convection phase until the oil reaches 450 degrees or more. Metal treated in this way cool rapidly and evenly. This reduces the risks of cracking, distortions, and uneven soft spots.
Water Quenching
Water quench hardening is typically used for low alloy steel grades that require a very rapid quench rate to achieve desired hardness.
Process for Water Quench Hardening Steel
The process of water quench hardening for steel is as follows:
- It begins by fully austenitizing the steel in the temperature range of 1500°F – 1650°F, depending on the steel grade.
- It is held at this temperature for a time commensurate with the part cross-section.
- This step can be done using a carbon-controlled protective atmosphere to prevent decarburization and the formation of scale as required.
- The load is then quenched in agitated water to produce a fully hardened martensitic microstructure.
- After the quench, the parts are tempered down to the specified hardness.
Equipments Needed
- Bench Vice
- Hand File

- Hack Saw

- Metallurgical Electron Microscope

- Metallurgical Polishing Machine

- Metallurgical Grinder

- Emery Paper

Procedures
Metallurgical Processes involved in this experimental procedure include:
- Cutting
- Filing
- Grinding
- Polishing
- Heat Treatment in a Furnace up to 910o C
- Grain analysis under an Electron Microscope
- Using the Bench Vice to firmly hold the sample (mild steel rods), carry out the cutting process using a Hack Saw. Cut 4 samples from the same mild steel rod.
- Filling of the spherical surfaces of the samples should be done after the cutting process, thus removing all vein stretches of cutting marks from the surface of the steel; this filling process is done using the Hand File.
- Furthermore, this process proceeds to using 220 and 320 gauges of Emery clothe in grinding the filed surface of the mild until a good polished, mirror-like, surface is obtained.
- Further smoothening polishing processes should be carried on the polishing machine until a truly mirror-like surface is finally obtained.
- Having completed 1 to 4 above, proceed to the Heat Treatment Furnace and there heat the 4 samples up to 910oC
- Afterward, carry out the Quenching; Quenching 2 of the samples in Oil and the other 2 samples in Water, thereby fulfilling the heat treatment processes.
- Further metallographic works in analyzing the result is carried out using an Electron Microscope and thus obtaining the grain properties of the heat treated samples.
Data and Results
Discussion of Results
To rightly analyzing the above data and result from the heat treatment of a mild steel rod, we will have to carefully relate the aggregate of ferrite to carbon for each type of quenching process and other necessary analysis respectively for both quenching processes. To starting with, let’s discuss the effect of carbon on commercially pure iron.
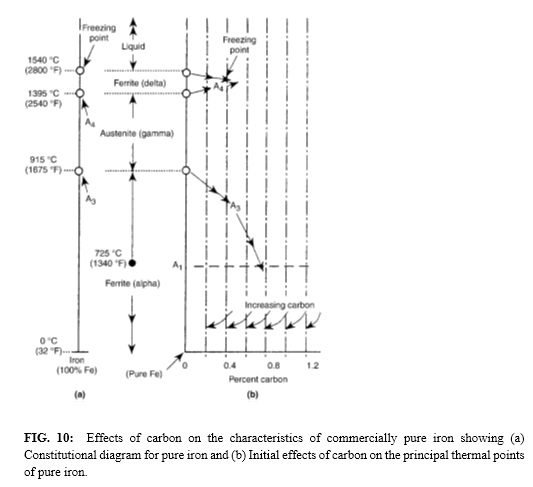
Iron-Cementite Phase Diagram
When carbon atoms are present, two changes occur (see Fig. 10). First, transformation temperatures are lowered, and second, transformation takes place over a range of temperatures rather than at a single temperature.
Phase
A phase is a portion of an alloy, physically, chemically, or crystallographically homogeneous throughout, which is separated from the rest of the alloy by distinct bounding surfaces. Phases that occur in iron-carbon alloys are molten alloy, austenite (gamma phase), ferrite (alpha phase), cementite, and graphite. These phases are also called constituents. Not all constituents (such as pearlite or bainite) are phases—these are microstructures.
A phase diagram is a graphical representation of the equilibrium temperature and composition limits of phase fields and phase reactions in an alloy system. In the iron-cementite system, temperature is plotted vertically, and composition is plotted horizontally.
Effect of Heat Treatment on the Mechanical Properties of the Steel.
Heat treatment involves the application of heat, to a material to obtain desired material properties (e.g. mechanical, corrosion. electrical, magnetic, e-t-c.). During the heat treatment process, the material usually undergoes phase microstructural and crystallographic changes. The purpose of heat treating carbon steel is to change the mechanical properties of steel, usually ductility, hardness, yield strength tensile strength and impact resistance. The electrical, corrosion and thermal conductivity is also slightly altered during heat treatment process.
The standard strengths of steels used in the structural design are prescribed from their yield strength. Most engineering calculations for structure are based on yield strength. Recent urban building in Nigeria have become taller for more effective utilization of above ground space and have increased in size and complexity through the adoption of new structural design. These trends have led to demand for steel of greater strength, good ductility and toughness. The special objectives of the research work are to carry out heat treatment process on locally produced rolled medium carbon steel, evaluate the effect of heat treatment processes on the mechanical properties such as tensile strength, ductility, toughness and hardness of the rolled steel and determine high strength, high ductility and low yield ratio of the rolled medium carbon steel.
Explanation of Some Selected Terms:
- Annealing, in metallurgy and materials science, is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a material above its recrystallization temperature, maintaining a suitable temperature for a suitable amount of time, and then cooling.
In annealing, atoms migrate in the crystal lattice and the number of dislocations decreases, leading to a change in ductility and hardness. As the material cools it recrystallizes. For many alloys, including carbon steel, the crystal grain size and phase composition, which ultimately determine the material properties, are dependent on the heating, and cooling rate. Hot working or cold working after the annealing process alter the metal structure, so further heat treatments may be used to achieve the properties required. With knowledge of the composition and phase diagram, heat treatment can be used to adjust between harder and more brittle, to softer and more ductile. - The Hardenability of a metal alloy is the depth up to which a material is hardened after putting through a heat treatment process. The unit of hardenability is the same as of length. It is an indication of how deep into the material a certain hardness can be achieved. It should not be confused with hardness, which is a measure of a sample's resistance to indentation or scratching. It is an important property for welding, since it is inversely proportional to weldability, that is, the ease of welding a material.
- Secondary Hardening: High-temperature hardness is made possible only by a very important straightening mechanism: secondary hardening, promoted by the precipitation of fine alloy carbides. The stronger the secondary hardening (meaning more intense carbide precipitation), the higher the tempering resistance of hot-work tool steels. Such precipitation intensity depends on the amount of alloy elements in solid solution, which is related to the alloy composition and heat treating practice obtained by molybdenum, vanadium, or tungsten alloying.
- Tempering is a process of heat treating, which is used to increase the toughness of iron-based alloys. Tempering is usually performed after hardening, to reduce some of the excess hardness, and is done by heating the metal to some temperature below the critical point for a certain period of time, then allowing it to cool in still air. The exact temperature determines the amount of hardness removed, and depends on both the specific composition of the alloy and on the desired properties in the finished product. For instance, very hard tools are often tempered at low temperatures, while springs are tempered to much higher temperatures.
- Work Hardening, also known as strain hardening, is the strengthening of a metal or polymer by plastic deformation. This strengthening occurs because of dislocation movements and dislocation generation within the crystal structure of the material. Many non-brittle metals with a reasonably high melting point as well as several polymers can be strengthened in this fashion. Alloys not amenable to heat treatment, including low-carbon steel, are often work-hardened. Some materials cannot be work-hardened at low temperatures, such as indium. However, others can only be strengthened via work hardening, such as pure copper and aluminum.
Conclusion
Generally, Heat Treated Steels (by Quenching) have high ultimate tensile strength and yield ratio of about 79% with excellent combination of impact strength, ductility and hardness which is very attractive for structural use.
Likewise, from the findings as related above, the steel developed by oil quenching is absolutely of greater advantage as they produce superior quality. Metal treated using Oil Quenching cools rapidly and evenly. This reduces the risks of cracking, distortions, and uneven soft spots.
References
- Practical Heat Treating, Second Edition: Fundamentals of the Heat Treating of Steel
- H.E. Boyer, Chapter 1, Practical Heat Treating, 1st ed., American Society for Metals, 1984, p 1–16
- Metals & Alloys in the Unified Numbering System, 10th ed, SAE International and ASTM International, 2004
- H.N. Oppenheimer, Heat Treatment of Carbon Steels, Course 42, Lesson 1, Practical Heat Treating, Materials Engineering Institute, ASM International, 1995.
- H.E. Boyer, Chapter 2, Practical Heat Treating, 1st ed., American Society for Metals, 1984, p 17–33
- Iron-Carbon Phase Diagram (a review): Callister Chapter 9 by University of Tennessee, Dept. of Materials Science and Engineering
- Effects of Heat Treatment on the Mechanical Properties of Rolled Medium Carbon Steel by O.O. Daramola, B.O. Adewuyi and I.O. Oladele. Extracted from Journal of Minerals & Materials Characterization & Engineering, Vol. 9, No.8, pp.693-708, 2010
- Annealing (Metallurgy) Wikipedia
- Substance and Technologies: Basic Heat Treatment Principles by Dr. Dmitri Kopeliovich
- Wikipedia. “Hardening (Metallurgy) “
- Wikipedia. “Tempering (Metallurgy)”
- Azo. "Heat treatment of steel processes"
- "The Benefits of Steel Heat Treatment". Specialty Steel Treating, Inc.
- Wikipedia. “Work hardening (Metallurgy)”
Credits: Timothy Alabi. Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria.